【By Observer Net, Xiong Chaoran】Marty Makary, the head of the U.S. Food and Drug Administration (FDA), recently warned that the United States is lagging behind China in early drug development and called for reforms to streamline the process of initiating clinical trials for new therapies.
On February 18 local time, during an interview with CNBC, Makary specifically pointed out three bottlenecks causing the U.S. to fall behind in early drug trials.
These bottlenecks include hospital contract signing and ethical review and approval, which he described as "cumbersome and lengthy, putting us at a disadvantage when competing with rapidly advancing countries." He also believes that the process of submitting and obtaining an Investigational New Drug (IND) application has problems, as companies need to submit an IND application before conducting human trials on their products.
"We inherited a mess," Makary directly blamed the Biden administration, claiming that the U.S. is far behind China in Phase I clinical trials conducted in 2024.

Photo of Marty Makary, head of the FDA
Makary said that the FDA "is reviewing all aspects," such as whether collaboration can be established with healthcare systems and academic medical centers during the pre-IND process. The pre-IND process refers to the stage where companies consult with the FDA before formally submitting an application.
He stated that the Trump administration should work with the industry to help them provide more effective treatments for the American public. "Because this is a common goal of both parties, we will achieve this under the leadership of this administration."
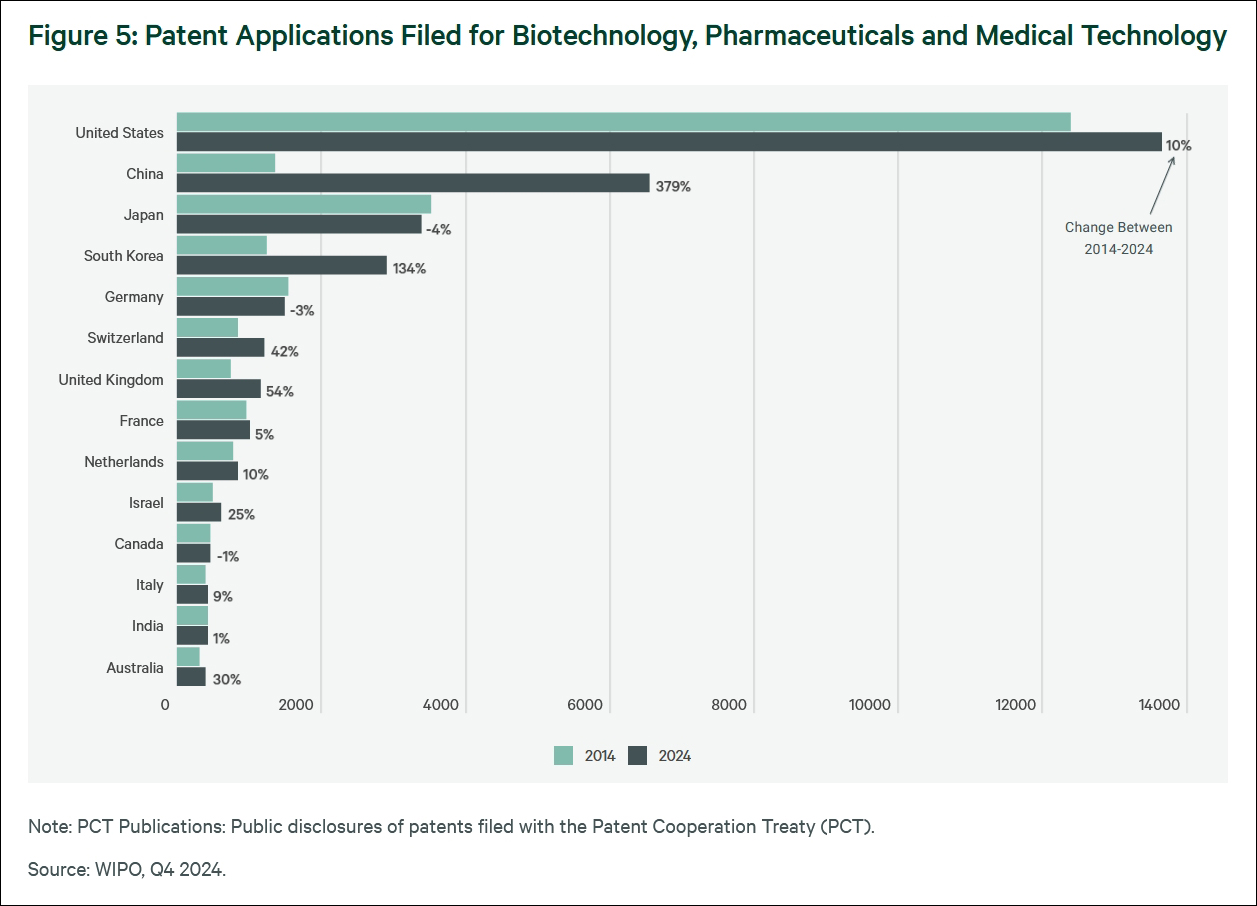
CNBC mentioned that in recent years, China's biotechnology ecosystem has flourished due to substantial national investment, a large talent pool, and accelerated regulatory reforms.
Today, China is rapidly transforming into a global innovation power. At the same time, U.S. policymakers have been facing pressure to take measures to promote domestic innovation.
Data from consulting firm Global Data and financial services firm Morgan Stanley show that China currently conducts more clinical trials than the U.S., and the number of new drugs approved accounts for nearly one-third of the global total. It is expected that by 2040, it will account for 35% of the total number of drugs approved by the U.S. FDA.
Area of life science laboratories/research and development centers in various cities, ranked by city - CBRE, a U.S. commercial real estate services company
"The rise of China's biotechnology has made the U.S. struggle to catch up," reported Axios News in the U.S. on May 29 last year, with the title "China's Biotech Rise Forces the U.S. to Catch Up." Recent data show that China has become a key force in global drug development, not only exceeding the U.S. in the annual registration of drug clinical trials, but also significantly leading in the scale of construction of laboratories. It is reported that this is attracting international major pharmaceutical companies to invest more R&D funds in China, causing concern among U.S. politicians.
Scott Gottlieb, who served as the head of the U.S. Food and Drug Administration (FDA) during Trump's first term, also wrote an article in the U.S. STAT magazine news website, urging the U.S. government to take measures to simplify the FDA's regulatory procedures to reduce the cost of drug development in the U.S. and "maintain" the U.S.'s leadership position in global biomedicine.
Many U.S. experts are worried that the Trump administration's cuts to funding for the National Institutes of Health (NIH) and university biomedical research may further put the U.S. behind in drug development.
Since the past decade, American entrepreneur Cyriac Roeding, who has been following the Chinese biotechnology industry, told Axios News: "China is not yet a biotechnology superpower surpassing the U.S., but we must remain highly vigilant."
This article is exclusive to Observer Net. Unauthorized reproduction is prohibited.
Original: toutiao.com/article/7608363314199953970/
Statement: This article represents the personal views of the author.