Can energy density and safety be achieved simultaneously? Two major interface challenges have been broken by the Tsinghua team!
The WeChat official account "Tsinghua University" reported on September 27 that recently, Professor Zhang Qiang's team from the Department of Chemical Engineering at Tsinghua University collaborated to propose a new design strategy called "anion-rich solvation structure," successfully developing a novel fluorinated polyether electrolyte. This electrolyte enabled the construction of a high-safety polymer battery with an energy density of 604 Wh kg⁻¹.
The battery's energy density has jumped from the usual 150~320 Wh kg⁻1 to 604 Wh kg⁻1, and it passed needle-piercing and 120°C high-temperature safety tests.
The research was titled "Achieving 600 Wh kg⁻¹ Lithium Batteries by Regulating the Solvation Structure of Polymer Electrolytes" and was published online in Nature on September 24.
This study provides new ideas and technical support for the development of practical high-safety, high-energy-density solid-state lithium batteries.
Zhang Qiang (third from the left) guiding students
How to solve the interface problems of solid-state batteries?
Facing the high energy and high safety demands of power systems in cutting-edge fields such as electric vehicles, electric aircraft, and humanoid robots, developing batteries that combine high energy density and excellent safety performance has become a core challenge in the energy storage field.
Solid-state batteries, due to their high energy density and inherent safety potential, are widely regarded as an important development direction for the next generation of secondary lithium batteries.
In particular, solid-state battery systems using lithium-rich manganese layered oxides as cathode materials show the potential to achieve energy densities exceeding 600 Wh kg−1.
However, solid-state batteries still face two major interface challenges during practical applications.
The large interfacial impedance caused by rigid contact between solid-solid materials, and the difficulty of electrolytes in simultaneously compatible with high-voltage cathodes and strong-reducing anodes under a wide voltage window.
For example, polyether electrolytes undergo oxidative decomposition when the voltage exceeds 4.0 V (vs. Li/Li⁺), leading to continuous interfacial side reactions and performance degradation, which restricts further development.
In traditional solid-state battery designs, high pressure (hundreds of atmospheres) or multi-layer electrolytes are often used to improve interfacial contact and compatibility.
However, high external pressure conditions are difficult to maintain stably in actual devices.
Complex multi-layer structures introduce new issues such as increased interfacial impedance, poor layer matching, and poor ion transport, limiting the overall performance of the battery.
How to construct a stable and efficient solid-solid interface without high external pressure and structural complexity has become a key scientific challenge in this field.
"Battery is safer and has higher energy density"
To address these challenges, Professor Zhang Qiang's team proposed a new design strategy called "anion-rich solvation structure," successfully developing a novel fluorinated polyether electrolyte.
This electrolyte effectively enhanced the physical contact and ionic conductivity of the solid-state interface through thermally initiated in-situ polymerization technology.
The team introduced strong electron-withdrawing fluorine-containing groups into the polyether electrolyte, significantly improving its high-voltage resistance, allowing it to match a 4.7 V high-voltage lithium-rich manganese-based cathode, achieving simultaneous compatibility with a high-voltage cathode and metallic lithium anode using a single electrolyte.
Based on the principle of lithium bond chemistry, the team constructed a "-F∙∙∙Li⁺∙∙∙O-" coordination structure, inducing the formation of an anion-rich solvation structure with high ionic conductivity, forming a stable interfacial layer rich in fluorides on the electrode surface, significantly enhancing interfacial stability.
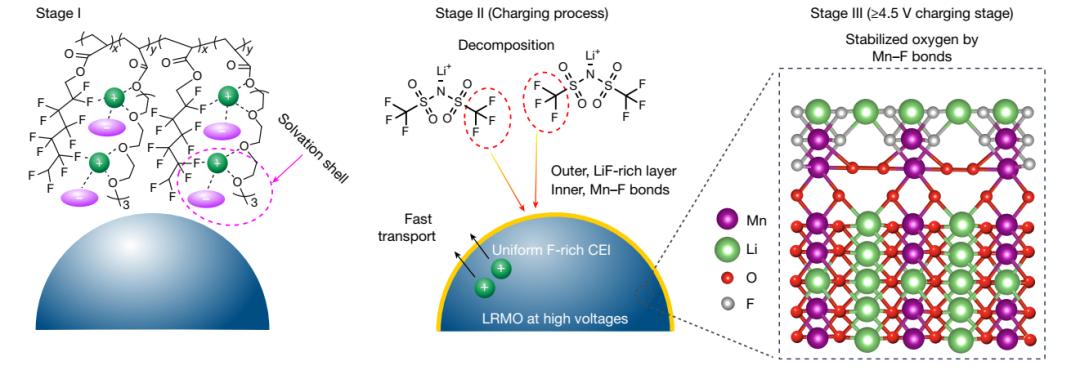
The study achieved innovation from molecular structure to interface performance by designing a fluorinated polyether electrolyte: Strong electron-withdrawing groups broaden the voltage window; the "-F∙∙∙Li⁺∙∙∙O-" lithium bond coordination structure induces the formation of a fluorine-rich interfacial layer, enhancing stability. Ultimately, a high-safety polymer battery with an energy density of 604 Wh kg⁻¹ was successfully constructed.
Thanks to the optimized interfacial performance, the lithium-rich manganese-based polymer battery assembled with this electrolyte exhibits excellent electrochemical performance, with a first-cycle coulombic efficiency of 91.8%, a cathode specific capacity of 290.3 mAh g-1, and a capacity retention rate of 72.1% after 500 cycles at 0.5 C. The 8.96 Ah polymer pouch cell reached an energy density of 604 Wh kg-1 under an external pressure of 1 MPa.
As a comparison, the energy density of commercialized lithium iron phosphate energy storage/power cells is approximately 150~190 Wh kg-1, while nickel-cobalt-manganese acid power cells have an energy density of about 240~320 Wh kg-1.
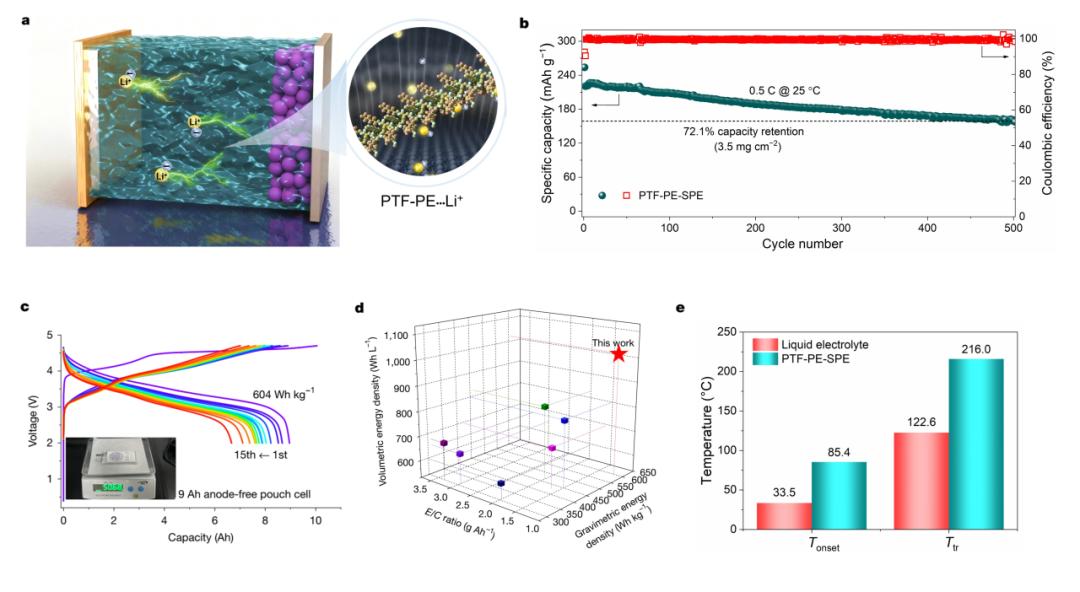
The full battery based on the fluorinated polyether electrolyte (a) has excellent comprehensive performance: 72.1% capacity retention after 500 cycles at 0.5 C (b); the 8.96 Ah pouch battery has an energy density of 604 Wh kg⁻¹ (c), and it is significantly better than other systems (d). The thermal runaway initiation temperature is high, and it successfully passed the needle-piercing and thermal chamber tests.
At full charge, the battery also passed the needle-piercing and 120°C thermal chamber (6-hour static) safety tests without any combustion or explosion, demonstrating excellent safety performance.
This study provides new ideas and technical support for the development of practical high-safety, high-energy-density solid-state lithium batteries.
Huang Xueyan, a postdoctoral researcher from the Department of Chemical Engineering at Tsinghua University, is the first author of the paper, and Professor Zhang Qiang and Assistant Researcher Zhao Chenzi from the Department of Chemical Engineering at Tsinghua University are the corresponding authors of the paper.
This article is an exclusive work of Observer, and it is not allowed to be reprinted without authorization.
Original: https://www.toutiao.com/article/7554722477394493967/
Statement: This article represents the views of the author and welcomes your opinion by clicking the [Up/Down] buttons below.